RMC-6236: A Curious Macrocycle
Macrocycles are a potent, intriguing class of therapies that are growing in importance. Revolution Medicines' RMC-6236 is a perfect example.
Revolution Medicines' RMC-6236 is an investigational new drug taking aim at advanced solid tumors. I'm excited and hopeful that this drug may have an impact for patients with otherwise poor prognoses. RMC-6236 also gives me an excuse to pen my thoughts about two areas of research I've been steeped in recently: protein-protein interaction (PPI) networks and macrocyclic peptides.
RMC-6236 is a macrocycle with a curious mechanism of action (MOA). It glues an innocuous protein (CYPA) to a family of known oncoproteins (RAS), creating a de novo PPI that disables downstream, oncogenic signaling. I'll first introduce PPIs and disease, survey macrocycle therapeutics, and combine these into a brief overview of RMC-6236.
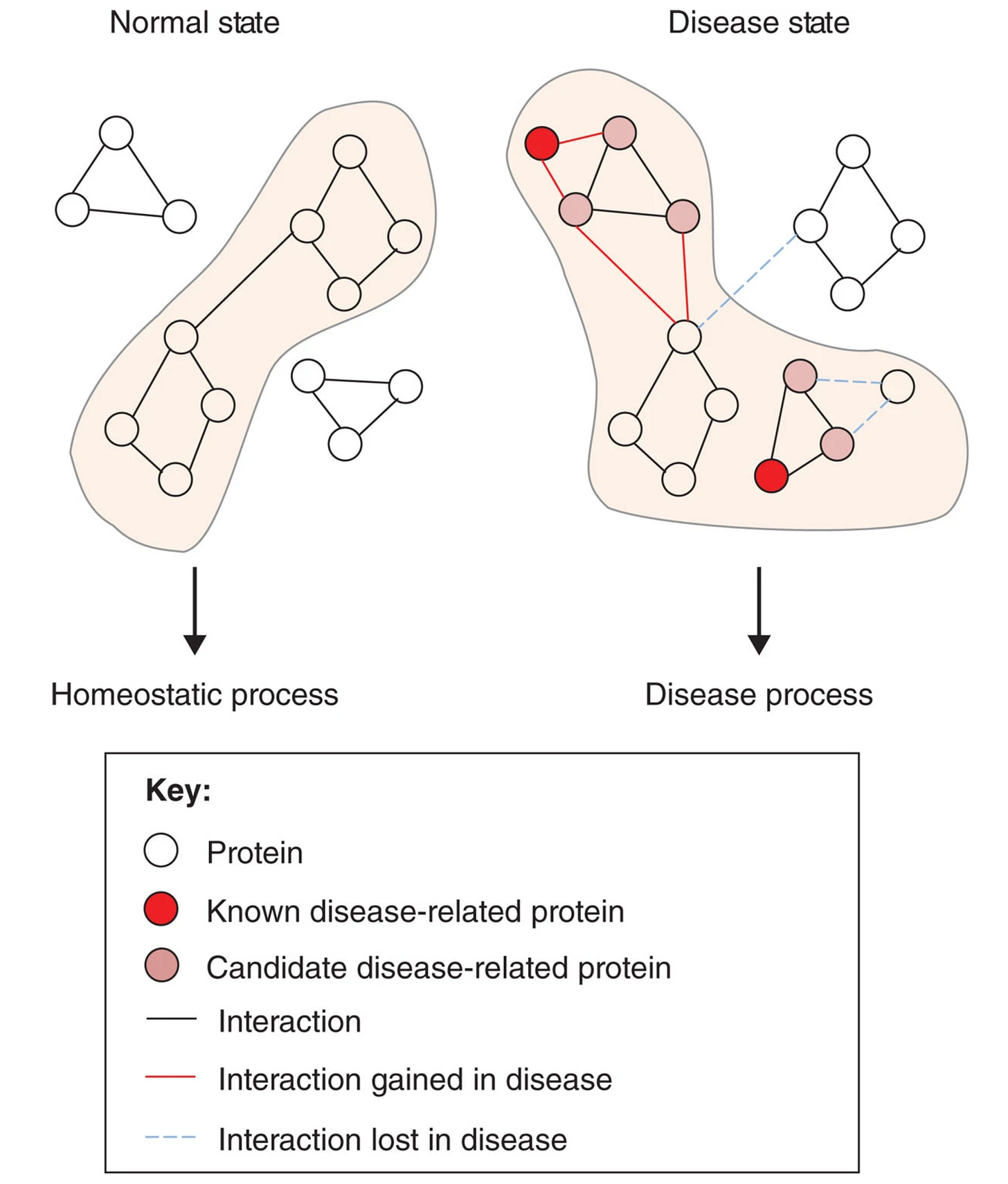
All of biology descends from molecules, like proteins, bumping into each other. Health and disease can be interpreted through the lens of PPI networks. Essentially, there's a natural order of PPIs that constitutes a healthy, homeostatic state in human beings. Disruptions in this pattern can be causal for or associated with a disease process. Therapies are our attempt at prodding a dysregulated PPI network back into a healthy shape via an external stimulus.
This partially explains why target discovery and selection are so challenging. Diseased networks create hundreds, if not thousands, of aberrant PPIs. How do we know which PPI is causal for the disease? If we intervene on the putatively causal PPI, is it enough to rescue the diseased phenotype? By affecting the disease-causing PPI, will unwanted and potentially toxic PPIs manifest?
These questions are a luxury because PPIs themselves can be extremely difficult to drug. Typically, when proteins interacts with each other, they do so using large, exterior surface patches. These hot spot regions are often smooth and flat, characteristics that make proteins notoriously challenging to develop small molecule drugs against. So how do we manipulate PPIs if not with traditional small molecules?

We can drug enormous interactions with enormous molecules. That's what biologics (e.g., antibodies) are for. Keytruda (pembrolizumab), one of the most valuable drugs on the planet, is a monoclonal antibody and PPI modulator. Specifically, Keytruda blocks interactions between the proteins PD-1 and PD-L1/PD-L2. These proteins all happen to be membrane proteins (receptors). This fact underscores one of the main limitations of antibodies. They can only drug extracellular targets.
Despite their inherent advantages such as high target specificity and in vivo stability, antibodies are simply too big to squeeze inside a cell. This is problematic when you consider that most proteins, and thus most PPIs, exist inside of cells. Besides the fact that intracellular PPIs are inaccessible to antibodies, there are other reasons why drugging a receptor may be suboptimal to rescue a disease process.
Receptors are often the first nodes of multimember, downstream signaling cascades. Kicking in the receptor is a good way to shut down all of this interior cellular circuitry. It's like CTRL-ALT-Deleting a large swath of PPIs. This may be effective, but could also cause unintended side effects.

Macrocycles are one exciting option to surgically modulate specific, intracellular PPIs. Macrocycles are peptides, a broad class of therapeutic that exists in the no-man's-land between small molecules and biologics. Peptides historically haven't made good drugs because they have poor in vivo stability. There's a renaissance happening with peptide drugs partly driven by the marquee success of drugs like semaglutide (e.g., Ozempic) which mimics an endogenous peptide hormone (GLP-1).
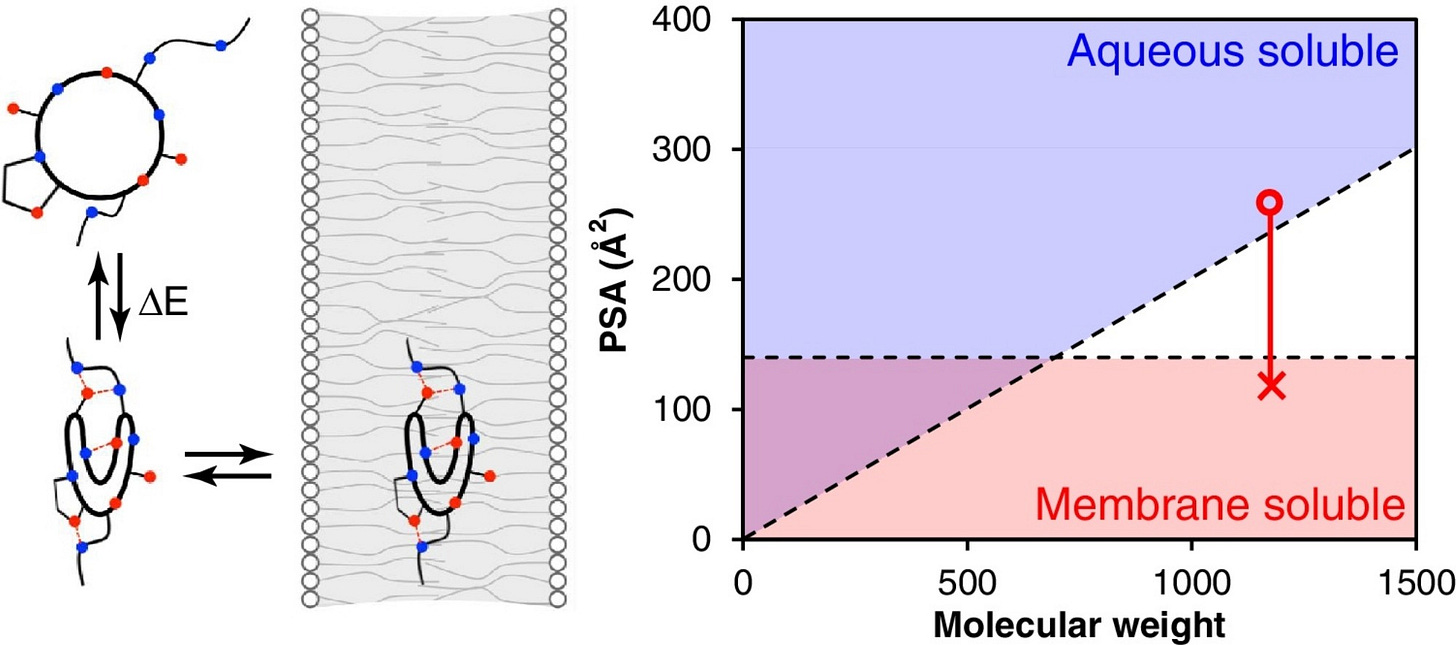
Unlike linear peptides, macrocycles have backbones that loops back on themselves to form roughly circular compounds decorated with functional groups. The circular shape imparts greater structural rigidity and defends against degradation by enzymes in the bloodstream. Macrocycles aren't rigid like a hula-hoop, through. They're more like rings of spaghetti. This flexibility is both a blessing and a curse.
Macrocycles exploit their conformational flexibility to become chameleonic, a quirky characteristic of compounds that exist beyond the rule-of-five. Chameleonicity means a molecule can adapt to its environment via conformational changes. Macrocycles can shield their hydrophobic residues, allowing them to become cell permeable despite their size.
Unfortunately, conformational flexibility is burdensome when screening and optimizing macrocycles. Virtual screening is a popular technique in small molecule drug discovery. Here, many designs are ranked in silico according to some scoring function. This function is often physics-based and can involve lightweight docking calculations. The forcefields involved generally aren't fast enough to deal with all the floppiness of macrocycles, greatly limiting the throughput of virtual screening campaigns.
Lead optimization can be tricky as well since macrocycles by their nature violate many cardinal rules of medicinal chemistry. Normally, med chemists want to make their compounds smaller, flatter, more linear, and more rigid. Macrocycles do the opposite.
Despite these challenges, macrocycle development seems worth it. Ostensibly, they're a readily manufacturable modality that can be made orally bioavailable, stable in vivo, with enough polar surface area to modulate intracellular PPIs in a precise fashion. RMC-6236 is a perfect example.
RMC-6236 is an oral macrocycle being purposed towards particularly aggressive solid tumors. Specifically, RMC-6236 targets a family of proteins called RAS. These are small GTPase proteins that regulate cell proliferation in response to growth stimuli. RAS proteins are commonly mutated in lung, colorectal, and pancreatic cancers. Mutations in RAS genes lock RAS proteins in their GTP-bound (ON) states, causing runaway signaling cascades that are drivers of tumor aggression.
Like many macrocycles, RMC-6236 was derived from a natural product (sanglifehrin A) which has high affinity to cyclophilin A (CYPA), a molecular chaperone. RMC-6236 acts effectively as a glue, but not as a degrader. Instead, this compound grabs CYPA and glues it to the exterior of RAS. Importantly, RMC-6236's landing zone is conserved across all RAS isoforms with equivalent potency, meaning that it hits the (ON) forms of KRAS, HRAS, and NRAS simultaneously. This could be crucial to overcome adaptive resistance whereby other RAS family members become upregulated in response to KRAS inhibition.
RAS proteins have proven difficult to drug, though several inhibitors do exist like sotorasib and adagrasib. One major challenge with KRAS inhibitors is durable efficacy. Unfortunately, KRAS often acquires additional mutations that can block inhibitor binding and cause therapies to lose effectiveness. RMC-6236 was designed to be robust against acquired mutations.
The formation of the ternary complex between RAS-RMC-6236-CYPA disables downstream signaling because it blocks the PPIs between RAS and other effector proteins. Because RMC-6236 lays down on a flat surface of RAS and not in a canonical binding site, acquired mutations are less likely to sterically occlude the binding between RMC-6236 and RAS, as shown below. This could increase the duration of response in patients.

Ultimately, the proof will be in the pudding. Though it's still early days, the clinical data produced by RMC-6236 has GI oncologists seemingly very excited. For example, let's look at some of the data in pancreatic adenocarcinoma (PDAC), a rather aggressive disease.
In a second line (2L), standard of care (SOC) setting, patients may only expect median 2-3 months of progression free survival (PFS) whereas RMC-6236 when given at low doses seems to show median 8 months of PFS in the 2L setting. Importantly, the curves for patients with more diverse RAS mutations was nearly identical, providing clinical evidence for the compound's robustness against resistance mutations.
The Phase 3 trial begins in the second half of 2024 with an expected PFS readout sometime in the first half of 2026. I'm eager to see the results and hope that its success catalyzes more research and development into therapeutic macrocycles and other PPI modulators writ large.
If you’re working in any of these spaces, send me a note!






Excellent depth here, and great read!
Interesting to see how one can begin thinking deeply about the properties of co-targets (e.g. cell type specificity, location, etc) rather than single targets as new technology expand capabilities of PPIs, induced proximity, Hetero-bifunctional targeted protein degradation, etc