Weekend ADC Musings
Antibody-drug conjugates (ADCs) are here to stay. But how much agreement is there as to how they work, why they're more effective, and why they're more tolerable?
Forward
Recently, I was reading through review papers on antibody-drug conjugates (ADCs). Specifically, I was interested in the mechanisms underlying efficacy and safety.
This is clearly a hot sector that’s pulled enormous amounts of deal-activity on behalf of established pharma and investors alike.
Despite being a few decades old, we’re seeing incredible efficacy results in-clinic. What’s interesting is that the debate around how ADCs work, why they’re ostensibly safer and more efficacious than their free diffusing cytotoxic counterparts, is still up for debate.
This isn’t a full think-piece, but rather some non-exhaustive bullet notes I took looking through these subjects. If you have a strong opinion about how groups can continue broadening the modality’s therapeutic index—I’m all ears.
Therapeutic Index (Efficacy)
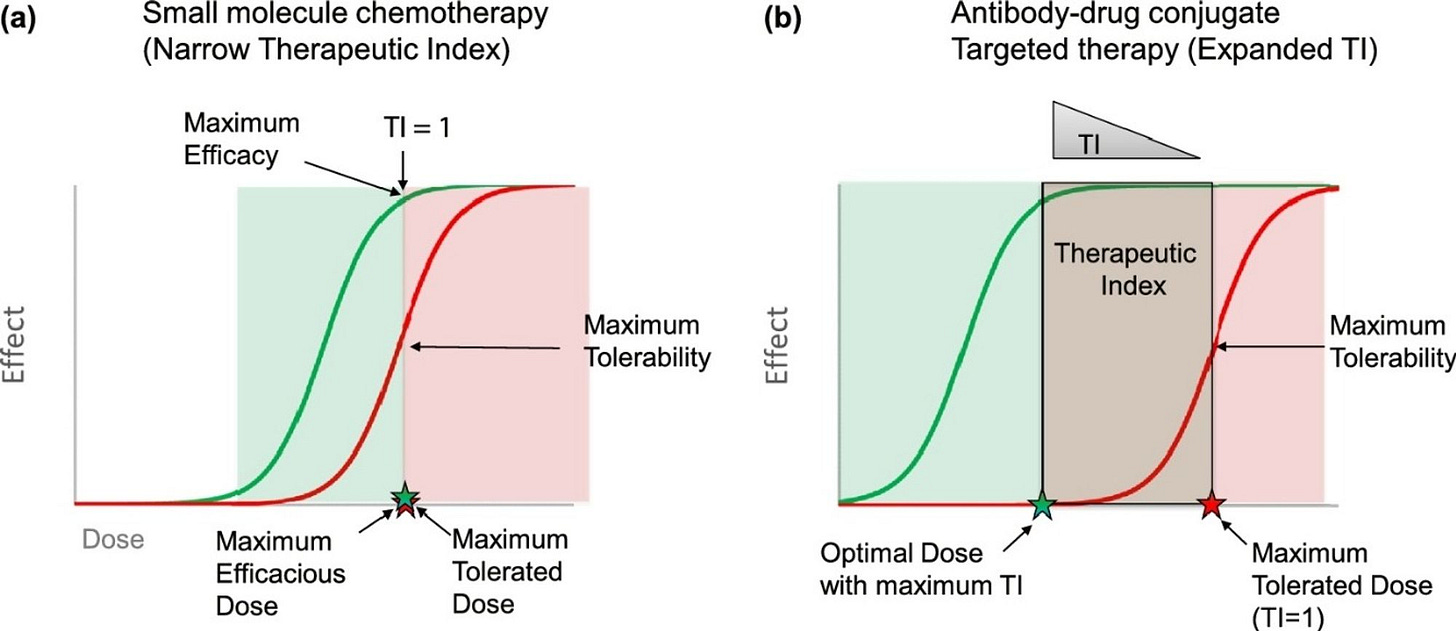
As mentioned, the central theory behind ADCs’ attractiveness is that the targeting agent broadens the therapeutic index by more selectively delivering the cytotoxic payload to cancer cells, as shown below.
I stumbled on an interesting debate between two papers (1, 2) centered on how to think about the therapeutic indexes of ADC drugs. I’ll present both.
Colombo et al. challenge the dogma that ADCs broaden the therapeutic index over free chemotherapy, suggesting that this hypothesis hasn’t borne out in the clinic.
The authors first cede that many ADCs confer a higher overall response rate (ORR) though not because the therapeutic index had increased. Shown below are ADCs (blue) and their free payload counterparts (pink) plotted to show clinical ORR across several solid tumor types.
Interestingly, the authors note that if you normalize the cytotoxin dose based on the drug-to-antibody ratio (DAR) (how many payload molecules are on each antibody) that the maximum tolerated dose (MTD) of the ADC-bound cytotoxin doesn’t differ that much from its free counterpart.
As shown in the chart below, the free cytotoxin dose (grouped by class) is displayed with hashed bars while the ADC-bound doses are in solid bars. Across payload classes, the normalized dose isn’t that much different than the free cytotoxin. The authors state this challenges the view that ADCs have higher MTDs, and thus, the theory that the therapeutic index broadens is murky.
To their credit, the authors then go on to describe other potential mechanisms for appreciable ADC efficacy beyond a higher MTD, including the substantially different PK of biologics compared to free drugs, the bystander effect, etc. The matter of PK is a great segue to a more recent paper that pokes around with this same issue.
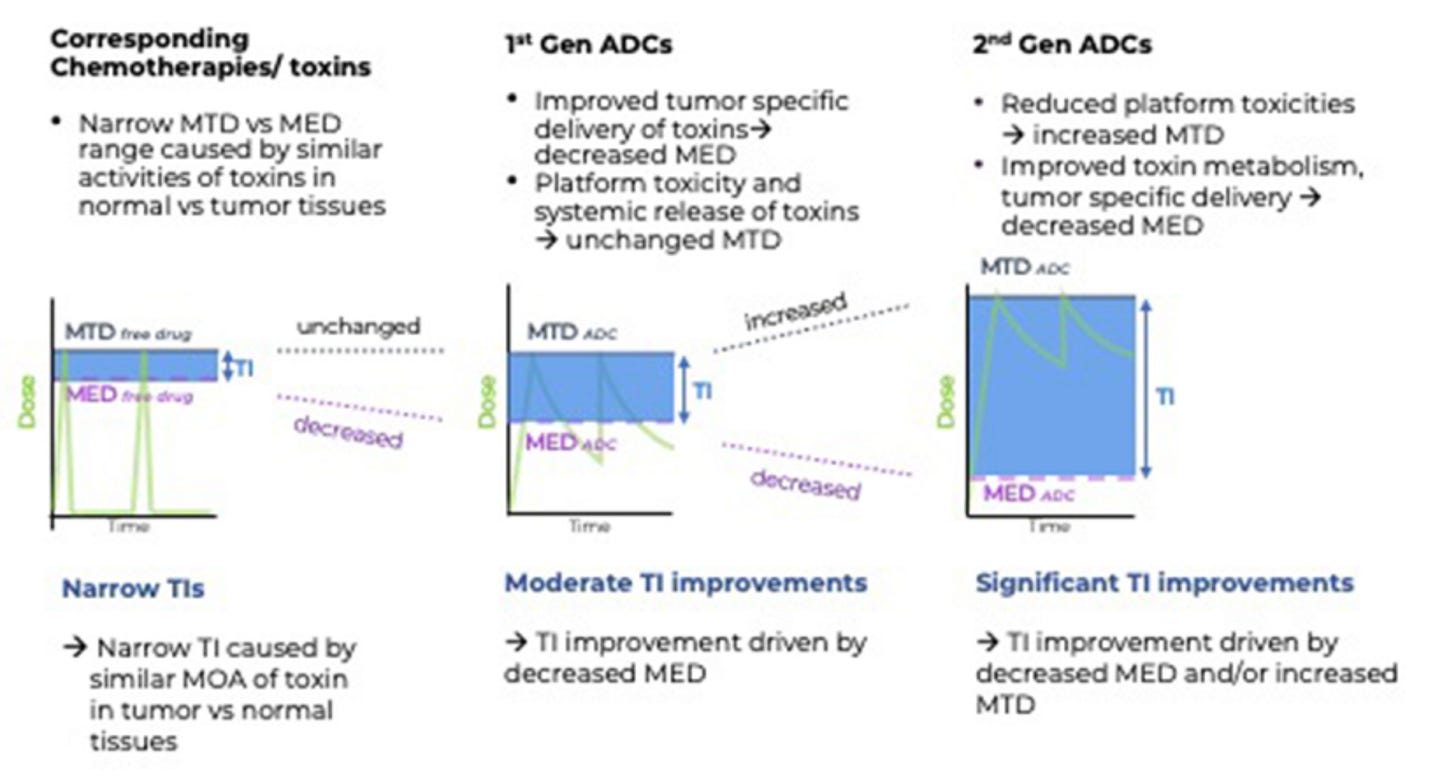
The second paper tackles the same issue of expanded TI, but instead focuses on the minimum effective dose (MED) rather than just the MTD. Moreover, they view TI through the lens of drug exposure kinetics.
The authors mention that while first-gen ADCs may not increase the MTD compared to their free drug counterparts, they do dramatically alter the drug exposure of the cytotoxin, which may help explain the increase in efficacy.
Recall that drug exposure relates to the area under the concentration v. time curve (AUC), which is a function of payload dose and clearance rate.
Freely diffusing cytotoxins have systemic exposure of minutes to hours whereas the bulky antibody component confers a half-life in the multi-day range. Therefore, even if the cytotoxin dose is equivalent (MTD) between the two modalities, the ADC-bound cytotoxin exposure is much higher—lowering the MED and expanding the therapeutic index, as shown in the chart above.
The moral of the story here is that while improvements in efficacy across solid tumor types and payload classes is very real, there’s still some disagreement as to what the underlying mechanisms are.
Safety and Toxicity
I looked through about 40 ADC companies founded after 2010 to see what they were focused on. Roughly half called out drug tolerability as the main obstacle holding ADCs back from reaching their true potential.
I also checked through 100 discontinued ADC trials and found that roughly one-third of them were nixed for safety reasons. Interestingly, 80% of all discontinued trials occurred in Phase 1, leading me to believe dose-limiting toxicities (DLTs) might’ve been common.
Researchers estimate only ~1% of an injected ADC dose actually contributes to killing cancer cells at the tumor site. The remaining molecules can contribute to toxicity.
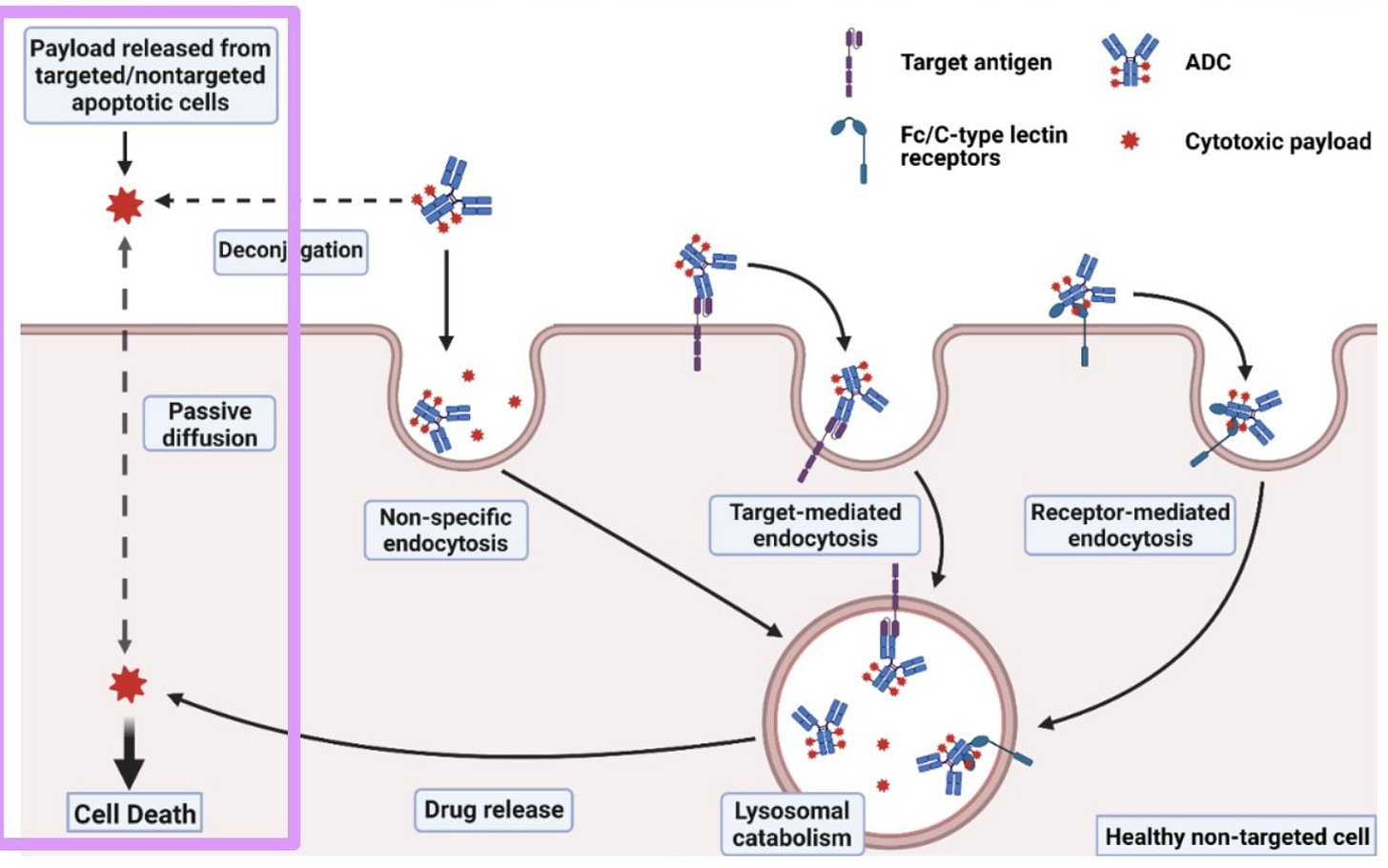
The following image presents several well-known ADC toxicity mechanisms, which I like to put into four bins (following image):
Several studies showed that the toxicity profiles of clinical ADCs were more closely correlated to the linker/payload than the drug target, suggesting that off-site, off-target toxicities are the prevailing mode.
Off-site, off-target toxicity stems from off-target endocytosis and/or premature payload deconjugation in plasma.
Off-target endocytosis may or may not be receptor-mediated. An ADC’s physiochemical properties (e.g., positive charge, high hydrophobicity) can cause non-specific endocytosis into healthy cells. In this case, no receptor is involved.
Conversely, on some types of cells, Fc receptors can interact with IgG (a standard ADC format), causing off-target, receptor-mediated endocytosis into healthy cells. A receptor is involved in this case.
Sometimes an unstable linker will break in circulation, freeing the ADC payload to passively diffuse into any nearby cell. This is generally more common for cleavable linkers than the non-cleavable variety.
Technically, drug exposure is calculated as the cumulative area under the curve (AUC) of Concentration v. Time, which is itself a function of the payload dose and the clearance (CL) rate.
Therefore, regardless of where the payload cleaves, the exposure shouldn’t change since CL processes are global. However, what does change is the ratio of on-site to off-site cytotoxicity. Premature payload deconjugation decreases efficacy relative to toxicity, narrowing the ADC’s therapeutic index.
Off-site, on-target toxicity is the usual boogeyman for antibody-based drugs. Because no drug target is 100% selectively expressed on tumors (beyond neoantigens), there will always be on-target binding on healthy cells.
Based on these examples, it’s clear that an ADCs toxicity profile is influenced by all components of the drug—the antibody, the linker, and the payload itself. Understanding how each component contributes to the molecule’s overall PK and ADMET properties is a critical tight-rope walk.
Looking across the roughly 40 companies I parsed through for this exercise, here are a handful of approaches to combat toxicity I thought were noteworthy:
Chase Agents—Develop a linker that is non-cleavable, except in the presence of a secondary compound. Following injection and subsequent plasma clearance of the ADC, administer the second agent to cleave the payload from the ADC.
Antibody Engineering—This is a broad category. Methods included building multi-specific antibodies that would only be internalized if bound to two cancer-associated antigens, increasing selectivity. Other methods included antibodies with pH-dependent properties (e.g., affinity) to control the fate of ADCs undergoing endocytosis—specifically to lessen the likelihood that they’re recycled back outside the cell.
Non-Antibody Scaffolds—Several groups are developing non-antibody binders such as peptides. Work in this space includes peptides that are protease resistant or have pH-dependent conformational ensembles that embed selectively in cell membranes only in acidic microenvironment.
Prodrug Masking—Attach small peptides near the binding regions of an antibody that mask its affinity. High levels of protease activity near a tumor will degrade the peptides, freeing the antibody to bind more selectively to tumor cells.
Inverse Targeting—Following ADC administration, flow in secondary antibodies that bind prematurely cleaved payloads in solution, removing them from contributing to toxicity via passive diffusion into healthy tissues.
Hydrophobic Masking—Engineer the linker molecule to shield the hydrophobic properties of most cytotoxic payloads, lessening the likelihood of non-specific endocytosis and ADC aggregation in plasma.